Month: October 2016
Quality Translations – Easy Insurance for Life Science KPIs

By Jack Fischer, Business Development Manager – Life Sciences division
If you work in the life sciences, then your job contributes to saving lives around the world. Diagnostics and other medical devices help physicians evaluate and prevent disease. Pharmaceutical products inhibit the progression of diseases. New and improved research tools broaden the scope of research outcomes and expedite the results upon which novel treatments are based.
With that said, there are two constants that life science professionals must face: the existence of patients beyond your initial market, and the need to convince your colleagues that certain risks associated with international initiatives needed to reach these patients can be mitigated. More often than not, your colleagues will have to convince others as well.
Across industries and around the world, 83% of consumers feel that having pre-purchase information in their own language is a critical factor in decision making.[1] When it comes to decisions about health, that number is even higher. High-quality translations performed by a specialized team represent a highly effective tool for reaching global health consumers and decision makers, as well as international stakeholders.
With the cost to gain 510k approval for a low-risk medical device commonly exceeding $31 million, and the overall cost to develop and win marketing approval for a new pharmaceutical product exceeding $1.3 billion, expanding your target patient populations is a financially attractive proposition[2], [3]. In order to manage the regulatory risk, and realize the potential of your international initiatives, clear communication across language barriers is imperative. Regulatory bodies must be absolutely confident about the methods and data you present to demonstrate the safety and efficacy of your treatment in your submissions. Physicians and patient community groups need translated education materials to inform productive & empathetic conversations about condition management.
When it comes to life sciences translation, it’s critical that your team or partnership be led by experts. Ensuring that your translation partner employs a highly-qualified team of medically-proficient linguists is critical to overcoming the language barrier. In-country linguists help incorporate cultural awareness that allows for a more fluid narrative, while retaining technical expertise. While cost-effectiveness is important, the potential loss of rejection for a regulatory submission or a clinical trial application far outweigh the cost of high-quality translations. Choosing a translation provider that can execute against these parameters can be the difference between approval and rejection for a regulatory checkpoint, enrollment in a clinical trial, compliance during audits, and adoption in your physicians’ offices.
While high-quality translations may seem like a small detail in the context of development costs, they are actually a key component to expanding your market and reaching more patients. They ensure the work of your regulatory, quality, and marketing teams are accurately represented on the international stage, allowing your company to reach your most crucial performance indicators with international stakeholders.
Going the extra mile to find a translations team that is experienced, informed, and committed to your success represents the best insurance you can buy for reaching patients and increasing revenues abroad. Plus, by helping share your treatments across borders, you can help improve countless lives.
[1] Common Sense Advisory, 2016
[2] Steinberg, D., Horwitz, G., & Zohar, D. (2015). Building a business model in digital medicine. Nature biotechnology, 33(9), 910-920.
[3] Joseph A. DiMasi, Henry G. Grabowski, Ronald W. Hansen, Innovation in the pharmaceutical industry: New estimates of R&D costs, Journal of Health Economics, Volume 47, May 2016, Pages 20-33, ISSN 0167-6296, http://dx.doi.org/10.1016/j.jhealeco.2016.01.012.
The Author
Jack Fischer
Jack Fischer is a Business Development Manager at Morningside’s Life Sciences division. After researching Tuberculosis genetics at Albert Einstein College of Medicine, and working with numerous biotechnology startups in New York, Jack moved to Morningside in order to apply his expertise to developing and implementing translation solutions for the life sciences industry. Jack has a dual B.A from Skidmore College in Integrative Biology & Business.
Get the latest insights delivered to your inbox
Cross-border Online Dispute Resolution: Language is Key

What is online dispute resolution?
Online dispute resolution (ODR) is a form of alternate (i.e., non-litigation) dispute resolution (ADR) that uses technology to facilitate the resolution process. As in alternate dispute resolution, ODR involves negotiation, mediation or arbitration, or a combination of the three.
While in ADR there are typically three parties (two disputants and the third neutral party), in ODR the technology itself becomes a fourth party that embodies its own range of capabilities – information organization, sending automatic responses, shaping written communications, monitoring performance, scheduling meetings, clarifying interests and priorities and so on. As technology advances we may even see the emergence of fourth party “avatars” that are created to judge disputes and become more skilled with each case. [1]
How does it work?
ODR has the potential to dramatically reduce the pressure on overburdened court systems by handling, for example, small-claims disputes or personal injury claims. But so far, only a handful of European countries (Switzerland, Austria, Norway) have succeeded in implementing ODR frameworks to scale. [2] For the most part, ODR is being used to handle commercial disputes in general and e-commerce disputes in particular.
A good example is Modria, a leading ODR cloud-based platform that provides retailers with a technology-mediated channel for settling disputes with consumers and thereby significantly reducing legal and customer service expenses. Modria’s customers create policies (or use Modria’s pre-configured policies) to automatically trigger actions in the resolution process including issuing a refund, sending a message, or routing to the right agent. The resolution process is initiated by the consumer directly from the retailer’s website and is guided step-by-step through the dispute resolution process. With one-click the customer can escalate to direct contact with customer service, and Modria also supports mediation and arbitration.
What happens when the dispute is cross-border?
Now that’s the (multi-)million-dollar question. The global e-commerce market is growing rapidly and cross-border e-commerce is becoming a significant percentage of that. Yet ODR platforms like Modria support English only.
Recognizing that for cross-border e-commerce to flourish, there has to be a convenient, multilingual dispute resolution mechanism, European Commission launched its official ODR portal in February. As you can see below, the consumer can initiate the ODR process in any one of the 23 official European languages:
Currently there is a note below the “Choose your language” message that “…you might be redirected to English content if a translation is not available yet.” One can only assume that, as the system scales up, more and more of the website will be available in the consumer’s native language.
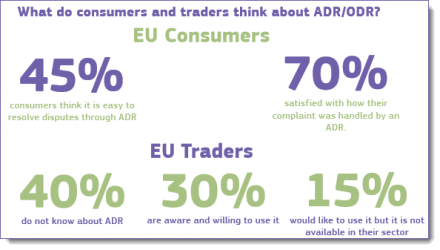 At this juncture, online traders are only obligated to provide a link to the ODR Platform on their website, and the ODR Platform will only automatically transfer the complaint to that entity if both the consumer and trader both agree on an ADR entity to handle their dispute. [3] The jury is still out as to whether this EU ODR initiative will be embraced by online traders and their cross-border customers. As shown in the info-graphic to the right [4], there is still work to be done to spread awareness, especially among traders.
At this juncture, online traders are only obligated to provide a link to the ODR Platform on their website, and the ODR Platform will only automatically transfer the complaint to that entity if both the consumer and trader both agree on an ADR entity to handle their dispute. [3] The jury is still out as to whether this EU ODR initiative will be embraced by online traders and their cross-border customers. As shown in the info-graphic to the right [4], there is still work to be done to spread awareness, especially among traders.
Cracking the language barrier is key to the proliferation of cross-border ODR
ODR industry thought leaders [5], [6] are hard at work designing models for global ODR systems that can reliably handle high volumes of ODR traffic within a labyrinth of diverse legal systems and across multiple languages. One suggestion is that the ODR process will be conducted in the language in which the transaction took place – or as determined by the online arbitrator. It also seems likely that being multilingual will be one of the threshold requirements for the online arbitrators. Perhaps open-source ODR translation databases can close some of the linguistic UI/UX gaps as well. The only thing that can be said with confidence today is that with technology advances and with the growth of cross-border e-commerce, robust multilingual ODR platforms will become a reality — sooner rather than later.
References


