Month: May 2017
Indian English 101

Is that girl eating your brain? No, she’s not a zombie. That’s Indian English for “is she bothering you?” The same words we use in the United States can have very different meanings in India, where English is spoken by over 125 million people. Some phrases are unique combinations of English words that are not used outside of India, while others utilize the same words but have a very different context.
Where did the unusual words and phrases of Indian English come from? Here is a brief history: The English language arrived in India in the early 1600s with the start of British colonization. Initially, Christian missionaries were the only English teachers around. Over the course of the next two centuries, English increasingly became the language of higher education, government administration, the media and the social elite. After Indian independence in 1947, India declared Hindi its official language. However, with 18 other national languages and hundreds of different dialects, English remained a popular second language. It was seen as a language of opportunity for those seeking social mobility, while simultaneously serving as a common language for Indians from different regions who spoke mutually unintelligible languages.
After nearly 400 years of use on the subcontinent, Indian English has morphed into its own unique dialect. Here is our brief introduction to words and phrases in Indian English that might look familiar, but have very different meanings.
Words or phrases that have different meanings in Indian English
- At the rate = the @ sign. Example: My mail ID is Sirena at the rate Morningside dot com.
- Belong to = am from. Example: Where are you from? I belong to Delhi.
- Crib = complain. Example: She won’t stop cribbing about her mother-in-law.
- Doubt = question. Example: Please Miss, I have a doubt.
- Flick = steal. Example: Someone flicked my phone on the train.
- Good name = first name
- Got fired = got yelled at
- Graduation = studied for a degree. Example: I did my graduation at the University of Delhi.
- Mail = email
- Mail ID = email address
- Mug up = cram or study intensively. Example: Tonight, I need to mug up for my final exam.
- Out of station = out of town
- Passed out = graduated. Example: I passed out of college.
- Saloon = hair salon or barbershop
- Visiting card = business card
Words or phrases unique to Indian English
- Airdash = to take a quick (sometimes emergency) flight
- Batchmate = a member of the same graduating class
- Convent-educated = educated in English (from when teaching used to be done by clergy members)
- Cooling glass = sunglasses
- Eating my brain = really bothering me
- Foreign-returned = having returned to India after studying or living abroad
- Freeship = full scholarship or payment for university
- Godown = a warehouse
- Incharge = a manager or supervisor
- Level best = very best effort
- Petrol bunk = gas station
- Prepone = reschedule something to an earlier date
- Rowdy-sheeter = a person who has a criminal record
- Sitting on my head = stressing me out
- Unmotorable = a road that isn’t suitable for use by motor vehicles
Get the latest insights delivered to your inbox
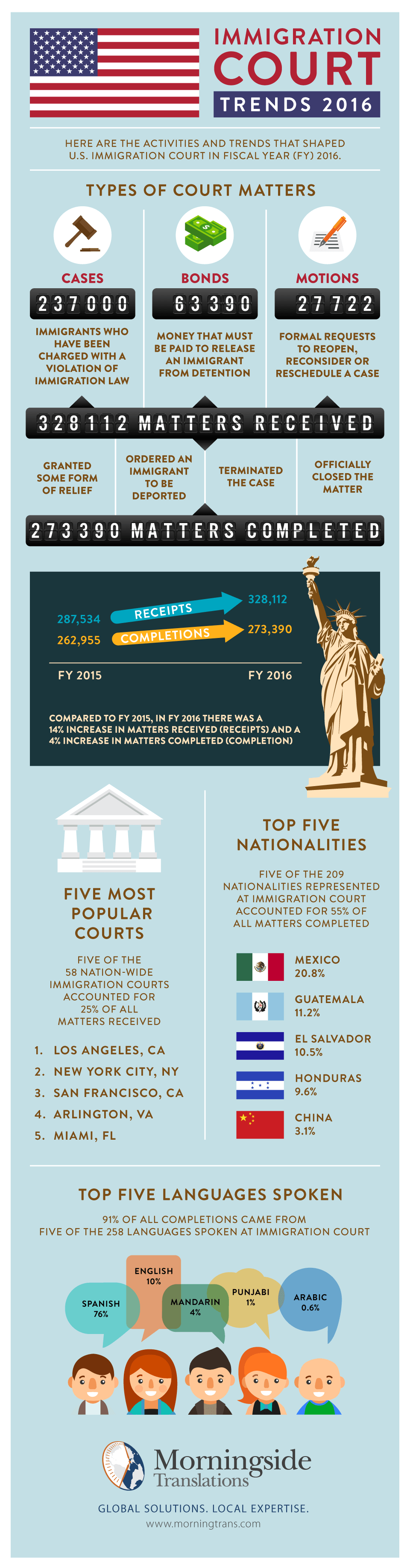
2016 Immigration Court Trends

Each year, the DOJ’s Executive Office for Immigration Review publishes a Statistics Yearbook on immigration court matters. The FY 2016 Yearbook was released last month, highlighting several significant trends. For example:
- 14% more matters were received by Immigration Courts in 2016 compared to 2015.
- 25% of all matters received came into the court systems of LA, NYC, San Francisco, Arlington (VA) or Miami.
- 76% of all matters completed were conducted in Spanish.
To learn more about key immigration court trends in 2016, check out our infographic below:
Get the latest insights delivered to your inbox
Translating Pharma Research During Global Clinical Trials
Virtually half of the clinical trials in life sciences today take place outside of the US, and a majority of trials regulated by the US Food and Drug Administration (FDA) are held abroad—mostly in developing countries.
One big reason for outsourcing clinical trials to developing countries is that it can save pharmaceutical companies as much as 90% on the cost of clinical staff at the testing facilities. Another important reason for outsourcing is to ensure that drugs are effective across different ethnic groups. The greater the diversity of the testing subjects, the more reliable the results will be for companies seeking to market their products to different populations across the world.
Testing pharmaceuticals on human subjects always requires great care and precision. When these tests are held in foreign countries with diverse languages and cultures, an entirely new set of challenges will arise for the study sponsor: All vital documents will require translation—either via a medical translator or a medical translation service.
Accuracy is critical
All of the collected information, including recruitment materials, informed consent forms, patient questionnaires, and case report forms must be translated into the local language and the results translated back into English to allow researchers to analyze the information.
This kind of translation leaves no room for error. If the forms are mistranslated, thousands of patient results could contain incorrect data. Regulatory submissions could become compromised, delaying review and approval. Every effort must be made to ensure error-free deliverables in order to maintain the integrity of the study.
Potential challenges
Two of the main translation challenges that arise when a test subject speaks a different language from the researcher are:
- Gaining conceptual equivalence — Infusing cultural meaning into translations. It’s not enough to simply understand the test subject’s language; a familiarity with their culture is necessary to convey the connotations behind specific words and phrases. This is particularly important when it comes to patient diaries, questionnaires, and other materials where understanding the cultural context of what the subject is saying is of utmost importance.
- Expressing intention — It’s vital to ensure that translations reflect what the subject intended to express. When a translation is too literal, it risks leaving out some of the intended meaning.
Industry guidelines
The International Society for Pharmacoeconomics and Outcomes Research (ISPOR) has released two reports on best practices for translations of:
Both reports address ways to improve translations and cultural adaptations in clinical trials. One key finding was the need for a separate process for medical translation from one language to another, versus same-language translations such as converting Spanish spoken in Spain to Spanish spoken in Argentina.
LSP assistance
Moving clinical trials overseas has helped pharmaceutical companies save money, increase diversity amongst research participants, and open new markets for industry products. The process, however, has placed translations in the spotlight. Using a professional medical translation service can remove the related stress by ensuring 100% accurate translations of clinical trials – no matter where they take place or in what language.
Get the latest insights delivered to your inbox
4 Fields That Require Professional Translation Services

Translations play a key role in global success for any business. No matter your industry, professional translation is crucial for a variety of purposes, ranging from marketing and HR to global clinical trials and e-discovery.
Given the connected nature of the international marketplace, it is no longer sufficient to restrict business materials to English, especially if you are looking to expand your brand and product overseas. Translating materials not only functions as a boon to accessing a regional market – in certain countries, translations are required to meet local regulatory and compliance mandates. Translations also offer spillover benefits, such as improving global workforce engagement.
Enlisting a professional language service provider (LSP) will allow you to enjoy all the advantages of accessing a global audience, and growing your international presence.
1. International Litigation
International litigation is highly complex on its own. Add in the language, cultural, regulatory and procedural differences, and the experience can begin to feel overwhelming. Regulations are susceptible to change, even on a single-word basis, and mountains of discovery materials often include foreign language documents. Unless these are all translated appropriately, the likelihood of a transnational miscommunication is high.
There are three instances, in particular, where legal translations come in handy:
- Cases heard in multiple courts, in different nations.
- Staff are not fluent in the necessary language for an international case.
- A law exists in another language, but is required for use or application.
Given the high level of specificity within law code, there is little-to-no room for error when it comes to translations. Therefore, it’s important to ensure translators are ISO-certified.
2. Marketing & E-Commerce
The proliferation of digital devices and solutions across the world have encouraged businesses to go global. Conducting business on a global scale means interacting with different languages and cultures while creating websites, brochures, advertisements, contracts, commercial agreements, quarterly and annual reports, etc. Whatever industry you’re in, communicating the proper message abroad is crucial.
This is perhaps the most apparent in the realm of ecommerce. At the beginning of 2016, reports showed that 57% of participants across six continents purchased an item from a website based overseas. By the end of that same year, the U.S. ecommerce market had accrued over $322 billion in revenue, with projections indicating sustained growth. To enjoy numbers like that, ecommerce businesses have to market to different regional audiences. With English spoken by only 26% of the world’s internet users, localization and translation have become critical tools for successful international business adaptation.
3. Global Clinical Trials & Research Publications
Translation takes a prominent role in the medical industry when it comes to conducting global clinical trials and publishing scientific papers.
- Clinical trials require an immense amount of paperwork. There are documents that have to be filled out by patients (informed consent forms), administering staff (case report forms), doctors (clinical outcome assessments), and more. If the research sponsor is conducting global clinical trials, then all of these documents need to be translated for each location – twice. The documents need to be translated from the original language into the local language of the test participants. Once everything has been recorded, it has be to translated back into the original language of the research sponsor. Throughout this complicated process, expert linguists should be on staff to answer questions.
- 75% of scientific papers are written in English, with some fields reaching as high as 90% in English. As such, non-English research must be translated in order to reach the rest of the scientific community.
4. Patent Filing
Filing a patent application anywhere is a highly complex process. It’s even more complicated when you’re applying for a patent in a different language under a different set of regulations. Similar to the legal industry, accuracy and compliance are critical due to the highly technical and precise language of patent applications.
Filing and maintaining a patent application in major international markets can range from around $11,400 in Israel to nearly $25,700 in Japan. As international filing increases, translation prices have also increased. According to the European Commission (EC), “the costs for a single translation of a patent may be more than €1500.” When you add the cost of translations in multiple languages and filing fees for multiple countries, the EC says “national validation costs can add up to about 40% of the overall costs of patenting in Europe.”
Working with a language service provider can help reduce costs and verify accuracy throughout the patent application process. In a recent IP Watchdog webinar on patent filing, Morningside IP’s Rob Bloom explained, “Since Morningside IP represents hundreds of clients, our foreign associates offer us a much more competitive fee structure than an individual organization with an IP portfolio could obtain.”
Work with an LSP from the start
Using a faulty or inaccurate translation in any of the above industries could lead to a lengthy and expensive repositioning of your international business. Instead, use a professional Language Service Provider from the start of your venture to seamlessly integrate the various regions of your global business.
Get the latest insights delivered to your inbox
The Right to Know: Translating Informed Consent
Informed consent is a basic human right. Therefore, risks must be made clear to participants of clinical trials or those undergoing surgical procedures. To ensure these basic rights are protected, the US Food and Drug Administration (FDA) mandates the use of informed consent forms (ICFs).
An ICF must include a range of information to minimize liability – and it must be written in a language that the subject understands. If the subject does not understand English well enough to give informed consent, the Institutional Review Board (IRB) mandates that the form must be translated by a medical translation professional. It is critical that the subject has a clear understanding of the risks and responsibilities of participating in a clinical trial.
Carrying out a procedure or clinical study without obtaining informed consent can expose medical professionals to a host of ethical challenges, malpractice suits or even charges of assault.
Defining informed consent
Informed consent is the process between a patient and a medical professional that provides the patient with the information necessary to determine the risks of undergoing a specific procedure or test. The informed consent process includes follow-up discussions to clarify ambiguities and ask questions, and should give patients ample time to make thoughtful decisions.
After the subject reviews the relevant information, he or she must sign the ICF. In cases where there is a reasonable expectation that non-English speakers will participate in a trial, the clinical investigator must submit the translated informed consent documents to the IRB before starting the consent procedure. After signing, the entire process comes under review again by the IRB, which is charged by the FDA to “protect the rights and welfare of subjects participating in clinical investigations.”
Elements an ICF must include
The following eight ICF components are required by the FDA – and must be written in the participant’s mother tongue or an officially acceptable translation – to earn IRB approval:
1. Description of Clinical Investigation – Subjects must be provided with an explanation of the research being conducted, its purpose, length of trial, and how it will be carried out (including any experimental procedures).
2. Risks and Discomforts – The subject must be informed of any foreseeable risks, danger or discomfort, including the severity of pain from a surgical procedure, recovery time, or even standard tests.
3. Benefits – The subject must receive a full description of how the experimental treatment could benefit them and/or others. The description should be clear, realistic and not overly optimistic.
4. Alternative Procedures or Treatments – The subject must be made aware of other options to treat their medical problem/s. The risks and benefits of those options must be fully explained.
5. Confidentiality – Subjects must be informed about who will have access to confidential medical records (e.g. the study sponsor), the degree of confidentiality they can expect, and under what circumstances the records would be turned over to a third party. In all cases, the FDA may inspect medical records (permission from the subject is not necessary).
6. Compensation and Medical Treatment in the Event of Injury – When there is more than minimal risk, subjects must be clear on what compensation and medical treatment, if any, may be obtained in the event of injury. Circumstances under which no compensation or treatment is available must be clearly expressed.
7. Contacts – Information must be provided regarding who to contact for further inquiries or complaints. The contact person should not be a member of the clinical team carrying out the trial so that the subject feels comfortable making a complaint or inquiry.
8. Voluntary Participation – Subjects must be made aware that they will not suffer any penalty or lose benefits they are entitled to by withdrawing from the study at any time. In addition, if withdrawal entails special procedures, those must be outlined in detail. It is important to note, however, that any data collected about a subject prior to their withdrawal is still fair game for inclusion in the study.
Other elements, when appropriate, should inform the subject of unforeseeable risks, circumstances under which the study leader may terminate participation, additional costs, and new, relevant research.
Exculpatory language is not permissible anywhere on an ICF form. This kind of language allows the individual to waive their legal rights, releasing the research sponsor (or its agents) from liability for malpractice or negligence (e.g. compensation for research-related injury).
The responsibility of ensuring informed consent
Responsibility for ensuring informed consent falls to three parties – the IRB, the clinical investigator, and the research sponsor. The three-pronged system ensures that the patient is protected from coercion or pressure to participate against his or her will. For example:
- A manufacturer wants to produce a new drug or medical device. They decide to sponsor a research project involving human subjects. They become responsible for “assuring the FDA that a study will be conducted in compliance with the informed consent and IRB regulations.”
- The sponsor hires a clinical investigator (and staff) and makes them sign specific forms (e.g. FDA-1572) or agreements promising to have the study reviewed by an IRB. From that point forward, the clinical investigator usually acts as the main point of communication between the IRB and the research sponsor.
- The IRB reviews all the materials to be signed by the research subjects, including officially translated versions of the ICF, before the consent process begins. Once everything is IRB-approved, it becomes the clinical investigator’s direct responsibility to collect ICFs from every research subject.
Translating for non-English speaking subjects
Ethically, scientific research should be carried out independent of considerations about the predominant language in a particular area. People who speak other languages should be neither targeted nor excluded. In order for foreign language speakers to make an informed decision, translation requirements dictate that all of the relevant consent forms must be delivered at the subject’s level of understanding (without medical jargon) in a language they speak fluently. For studies that pose more than minimal risk to subjects, ICF translations must be performed by a professional medical translator or a qualified Language Services Provider (LSP) that relies on medical translation professionals.
When to use a qualified LSP
A qualified translator must be retained if:
- A subject does not understand English but is literate in other languages. In this case, a written ICF must be provided in a language that the participant understands and a translator must be provided who is fluent in English and the participant’s spoken language.
- A subject does not understand English and is illiterate in other languages. In this case, an oral explanation must be given in the subject’s language. A witness must be present, and a short form in the subject’s language and a detailed long form on the oral presentation are required.
The essence of personal autonomy
Medical trials go to the very heart of personal autonomy. The law ensures that no one should ever be subject to medical experimentation without full understanding of the risks, benefits, alternatives, and procedures.
The FDA provides clear directions for ensuring that the necessary information is presented in an understandable manner. To minimize liability, clinical trials must adhere to the FDA’s guidelines as closely as possible, including ensuring that informed consent forms are handled by medical translation professionals.

