Month: July 2020
All You Need to Know About Text Expansion and Contraction

You may have noticed that it can take more words to say something in one language than in another. For example, it takes three words in French (s’il vous plaît) to accomplish what you can with one word of English (please). Text expansion and contraction has a direct impact on the cost of translation, and it plays an extremely important role when considering design for multilingual websites, marketing campaigns, presentations – and pretty much any project that needs to be available in multiple languages.
What is text expansion and contraction?
This is a term used in the translation industry to account for the increase or decrease in a document’s final word count when it is translated. For example, French and other romance languages are known to be wordier than English. A document with 1,000 English words translated into French will convert into approximately 1,150 target words — a 15% increase in the document’s word count. That is text expansion. Asian languages like Chinese, Korean and Japanese will usually convert into fewer total words when translated into English. That is text contraction.
How does text expansion and contraction affect pricing?
Calculating expansion or contraction during translation is not an exact science. Most language service providers use ratios based on typical expansion/contraction rates for different language pairs. For example, in English to German translation, the word count usually contracts by 20%, and therefore this is the ratio that most translation companies use. (If you have ever seen a German compound word like Donaudampfschifffahrtsgesellschaftskapitänsmütze – which translates to “Danube steamboat shipping company Captain’s hat” – you’ll understand why.) Pricing can also be affected by a document’s subject matter, terminology, and the quality of the original writing — all of which can cause the text to expand more or less than the ratios predict.
How does text expansion and contraction affect design?
Let’s say you have a short product description in English (100 words) that needs to be translated into French for a PDF brochure, a video advertisement, and a software app. Here’s what could happen: A one-line English headline in a cleanly laid out PDF brochure can turn into a two-line French headline that bumps the rest of the copy down or even off the page. A video with music and graphics synced to English text can become unsynced when the text is translated to French. The menu buttons on a software app can end up expanding awkwardly or displaying incomplete words, causing a major UI problem. So, as you can see, translations that result in text expansion or contraction can have a very real impact on design quality. That’s why it’s important to work with a localization expert to ensure all of your materials are not only translated accurately, but visually adapted as well.
Trust a localization expert
Morningside has 20 years of experience working with businesses to linguistically adapt products, documents, websites and software applications for target markets around the world. It’s a process we call localization, where translation is just the first step. We then work to adapt layout, design and graphical elements to properly fit the translated text. Our team includes expert linguists, DTP specialists, voiceover talent, and dedicated project managers experienced in providing multilingual localization services in 200+ languages. If you have any more questions about text expansion and contraction, or localization in general, contact us below.
Get the latest insights delivered to your inbox
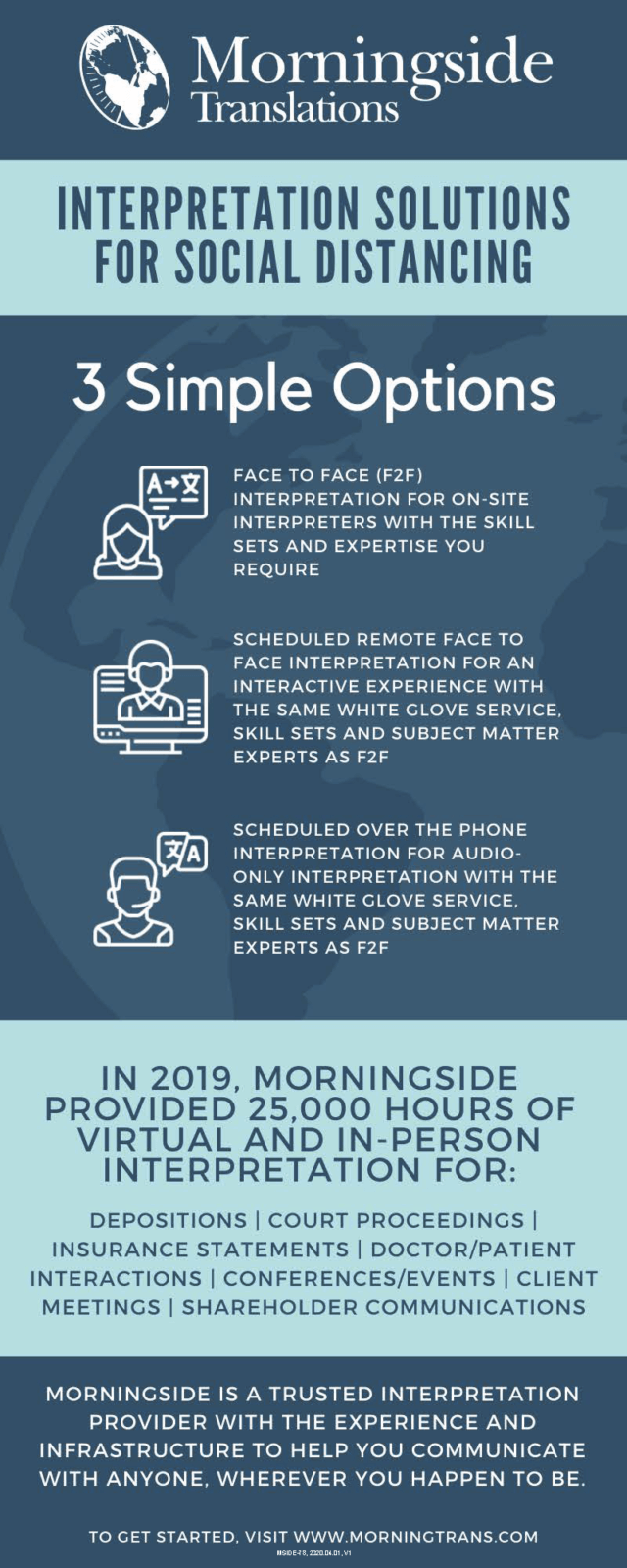
3 Simple Interpretation Solutions for Social Distancing

Over the course of months of worldwide social distancing efforts, inquiries regarding Morningside’s interpretation capabilities have increase dramatically and we’ve seen a shift in the way that clients are utilizing our services. We understand that our clients need greater flexibility during this time, and want to confirm that Morningside’s interpretation capabilities are readily available to you for depositions and court proceedings, as well as mission critical business functions. You can read more about them below.
Get the latest insights delivered to your inbox
Conducting International Clinical Trials During COVID-19

With people everywhere anxiously awaiting word of an effective treatment or vaccine for COVID-19, clinical trials have never before been so closely watched by so many. There are, according to one count, over 1700 novel coronavirus-related clinical trials underway around the world as of this writing.
Fast track approval
When a drug is urgently needed for a serious, currently untreatable disease, such as COVID-19, it may be possible to gain “Fast Track” approval, an emergency-case designation awarded as necessary by the FDA in the U.S and by other nations such as China and the U.K.
This recently occurred with Gilead’s drug remdesivir, which had already gone through clinical trials for treating Ebola. The Ebola trials ultimately proved disappointing for remdesivir’s efficacy in fighting that disease, though there were no questions regarding its safety. When COVID-19 arrived, Gilead revived remdesivir as a drug candidate because it did seem able to inhibit SARS-CoV-2 in vitro.
Granting fast-track approval for a COVID-19 drug that was known to be safe was an easy choice for the FDA, and remdesivir was made available for use by emergency physicians treating COVID-19 patients. The results, however, are mixed: While some limited data suggests remdesivir may shorten the duration of the illness, a large Chinese study of patients with more severe cases of COVID-19 found no statistical benefit from the drug.
Clinical trial documents
Pharmaceuticals are, of course, a field where the smallest details matter, and nuance can be everything. One of the trickiest, most critical, parts of international clinical trials is making sure that everyone involved shares a common understanding of the clinical trial process and its findings. That is why clinical trial document translation is required by law across the globe.
Every trial participant must be able to access all materials in their native language in order to avoid errors and invalid results. It’s also important to translate all documentation for those who will be planning, administering, and conducting the trials. This is the best way to ensure that no misunderstandings occur between parties operating in different regions, and that important implications and subtleties in one language are faithfully communicated to everyone involved.
How an LSP can help
When clinical trials are conducted internationally, clinicians, patients, sponsors and regulatory bodies must be able to communicate easily and with a high degree of accuracy. Engaging a qualified language service provider (LSP) such as Morningside increases the likelihood of better outcomes, ensuring that multilingual communication never stands in the way of a trial’s productive completion.
A good LSP can also help to quickly move a study forward by providing rapid turnaround of the necessary translated documentation, allowing medical research teams to meet critical regulatory deadlines. With the added pressure of finding a vaccine for COVID-19 as quickly as possible, quick turnaround is more important now than ever before.
If you are involved in COVID-19 clinical trials, feel free to contact us about how to achieve the best translation results. We are experts at translating and validating a wide range of clinical trial documents — from informed consent forms (ICFs) to questionnaires, COAs of all types and case report forms (CRFs). Our team of linguists consistently deliver accurate, culturally adapted translations for clinicians, review boards, and regulatory bodies.

