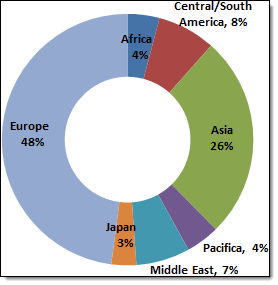
Life science companies – including 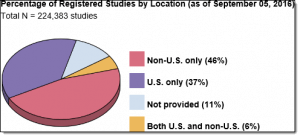 medical device, biotech and pharma – are increasingly turning to countries outside the US and W. Europe for clinical trial sites. According to the statistics kept by the National Institutes of Health, almost half of the currently registered studies are being conducted entirely outside of the US and an additional 6% are being conducted both in and outside the US. A more detailed breakdown of the distribution of the non-US multi-regional clinical trial (MRCT) locations indicates that Europe accounts for slightly less than 50% of clinical trials, followed by Asia with 26%, Latin America with 8% and the Middle East with approximately 7%. [1]:
medical device, biotech and pharma – are increasingly turning to countries outside the US and W. Europe for clinical trial sites. According to the statistics kept by the National Institutes of Health, almost half of the currently registered studies are being conducted entirely outside of the US and an additional 6% are being conducted both in and outside the US. A more detailed breakdown of the distribution of the non-US multi-regional clinical trial (MRCT) locations indicates that Europe accounts for slightly less than 50% of clinical trials, followed by Asia with 26%, Latin America with 8% and the Middle East with approximately 7%. [1]:
Why are companies going abroad?
The two major benefits of MRCTs for the life science companies are: access to larger and more diverse patient populations that are often willing participants thus expediting enrollment; and the lower cost of conducting trials in developing countries. One publication estimates that, for a 60,000-subject study, $600 million per year can be saved by shifting half of the late-phase trials from the US and Western Europe to South America or India. [2]
The other benefits of the growing trend toward MRCTs are improvements to the healthcare and clinical research infrastructures in the target countries; opportunities for talented practitioners to be principal investigators; faster launches of innovative drugs and devices and improved global patient access to these products.
What are the challenges of going abroad?
At the clinical level, differences in medical practice and standards of care across multiple geographies can make it difficult to validate the results. At the clinical study design level, diverse and sometimes contradictory regulatory requirements have to be bridged regarding issues such as endpoint definitions and required levels of evidence.
There are also potentially troubling ethical issues such as adequate protection of research subjects, getting truly informed consent, levels of research conduct and transparency, the quality of the local reviews that authorize the research, etc. [3] In response to unease that is frequently expressed concerning how clinical trials are conducted in developing countries, the large pharma companies, such as Pfizer, have made clear and public commitments to ensuring that their research strictly conforms to international standards no matter where the trial is taking place.[4]
Translation can make or break an MRCT
Clinical trials are highly structured and meticulously documented processes. A partial list of the documents that have to be made available in all relevant languages for healthcare professionals, investigators and patients at every clinical trial site includes: Clinical Study Protocols, Case Report Forms, Investigator’s Brochures, Patient Information Leaflets, Patient Informed Consent Forms, Patient Questionnaires, Patient-Reported Outcomes.
Terminology has to be stringently controlled across all languages. Translations have to be done by qualified professionals under strict quality assurance processes, such as validation by experienced clinicians. Errors not only potentially endanger lives, they can undermine the very validity of the trial. To this end regulatory bodies such as the FDA, EMA and professional organizations such as ISPOR (International Society For Pharmacoeconomics and Outcomes Research) provide good practice guidelines on how to conduct and document MRCTs so that patients are protected, their reported outcomes – including Adverse Events — are unequivocally validated, and the clinical trial data can be used confidently to make a decision about whether or not a new medication or device should be allowed to enter the market.
References


