Morningside is the fastest growing major language service provider in North America and one of the largest IP service companies in the world. Specializing in patent, life sciences, and legal services where accuracy and subject matter expertise are paramount, Morningside provides ISO-certified translations into more than 150 languages and offers a suite of technology-enabled localization and IP management solutions. Morningside is the trusted partner to thousands of organizations including Fortune 500 companies, Am Law 200 firms, and international regulatory bodies. Headquartered in New York City, Morningside has offices across the globe in San Francisco, London, Hamburg, and Tel Aviv.
Year: 2019
Pros and Cons of Provisional Patent Applications

As of June 8, 1995, the USPTO has offered inventors the option of filing a provisional patent application, which was “designed to provide a lower-cost first patent filing in the United States.” One of the key benefits of this option is that filing a provisional patent does not trigger the start of the 20-year patent term.
What is a provisional patent application?
A provisional patent application is often described as a placeholder for a future, non-provisional application that is based on the invention described in the provisional application. In contrast with a non-provisional application, a provisional application is exempt from:
- an inventor’s oath or declaration;
- the duty to disclose information material to patentability (prior art); and
- formal patent claims.
A provisional patent application will not be examined or disclosed by the USPTO because a provisional application cannot become a granted patent.
Then why should I file a provisional patent application?
The filing date of a provisional patent application establishes the priority date for a non-provisional patent application that claims the benefit of the invention described in the provisional application – so long as the non-provisional application is filed within 12 months of the provisional filing date.
During the 12-month period, the inventor can file additional provisionals that further elaborate on the invention – all of which can be included in the non-provisional application (with the oldest providing the priority date).
Be the first to file
When the America Invents Act came into effect in March 2013, the US patent process shifted away from its historic “first to invent” approach and adopted the globally practiced “first to file” system. Being able to establish a priority date for an early-stage invention is probably the greatest benefit of a provisional patent application, especially since the provisional application has no impact on the future patent’s 20-year term.
The expense of preparing and filing a good provisional application is about half the cost of preparing and filing a non-provisional application. With the priority date secured, the inventor now has 12 months to further develop the invention and validate its market potential before incurring the expense of filing a non-provisional patent application and seeing it through the patent examination process.
In the meantime, the inventor can use “patent pending” to describe the invention, enhancing its perceived value in the eyes of potential investors and partners.
Things to watch out for
A non-provisional patent application that references a provisional application must be based on the invention as it is described in the provisional application. Thus it is important to write the provisional patent application with great care, anticipating as many ways as possible of practicing the invention so as not to restrict the future non-provisional patent application (which cannot be changed so easily after filing).
Provisionals are often filed because the inventor wants to share the inventive solution with potential investors or partners as quickly as possible and seeks to protect it before showing it to others. It is important, however, not to fall into a false sense of security. Even a non-provisional patent application’s claims cannot be enforced until the patent has been granted. A provisional patent application affords even less protection.
IP trends
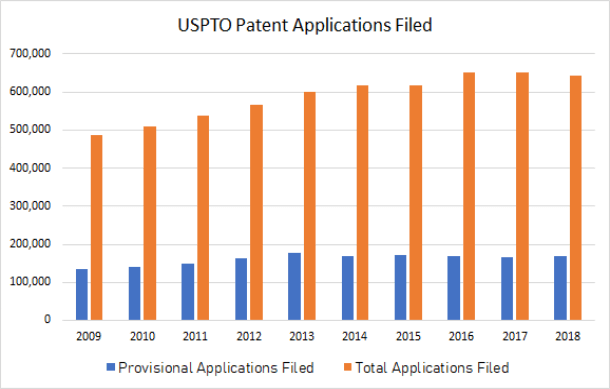
The graph below shows the number of provisional vs. non-provisional applications filed in the past decade (from 2009 to 2018), as taken from the USPTO’s annual workload tables. The number of provisional applications has increased 26% in that time, from just over 134,000 to just under 169,000. This upward trend indicates strong interest in leveraging provisional applications as part of an overall IP strategy.
An easy out
A final benefit of provisional applications is that they give the inventor a window of opportunity to further test the technological and commercial feasibility of an invention. If further testing yields poor or unprofitable results, the project can be dropped before committing to a long and potentially expensive non-provisional patent application process.
Get the latest insights delivered to your inbox
Labeling Requirements for Medical Devices in Israel

Regulatory requirements for the medical devices industry are constantly changing, which can be quite challenging for manufacturers and distributors that need to comply with a variety of country-specific registration requirements.
If you wish to sell a medical device in Israel, you’ll need to demonstrate compliance with the requirements of the Medical Device Division of the Israeli Ministry of Health (AMAR).
Israel does not have its own classification system for medical devices, but it accepts the classification method and the applicable regulatory requirements of the United States, EU, Australia and Canada. As such, products that are FDA approved or EU CE marked should already have the essential parts of the device dossier required to complete the registration process in Israel.
US Food & Drug Administration (FDA)
High quality labeling is necessary to ensure device performance as well as patient and user safety. As such, the use of FDA recognized labeling standards is highly recommended and can facilitate your approval/clearance process. FDA labeling requirements can be found in Title 21 (see Sec. 801.15). You may also refer to ISO 15223-1, an international standard recognized by the FDA for use of symbols on medical device labeling.
Note: The FDA constantly publishes new guidelines which can assist companies to better understand the regulatory requirements, so be sure to reference the most current information available.
EU CE marking
A CE mark is a specific certification demonstrating that a product meets EU health, safety and environmental requirements. In order to apply a CE mark on a medical device, you must demonstrate compliance with the EU Medical Device Regulation (MDR) which defines labels as “written, printed or graphic information appearing either on the device itself, or on the packaging of each unit or on the packaging of multiple devices.” The MDR requires that labeling will be provided in plain language that is clearly comprehensible to the end user.
Israeli guidelines
As stated above, if a product is already in compliance with the FDA or EU MDR then it can be assumed that its labeling covers the regulatory requirements in Israel. Still, there are some specific requirements related to labeling translation in Israel. For example, the Israel Ministry of Health has different translation requirements for devices intended for professional use and devices intended for home use.
In Israel, when the device is intended for professional use in healthcare facilities, the labeling may be provided in English-only. When the device is intended for home use, labeling must be provided in three languages: Hebrew (Israel’s official language), Arabic and English.
In summary
The Medical Device Division of the Israeli Ministry of Health (AMAR) is the regulatory authority in Israel for medical devices. In order to register a device in Israel, you must submit a product dossier (including labeling and regulatory approvals from other recognized countries) to AMAR. In Israel, labeling of products for professional use can be provided in English-only, while labeling for home use devices should be provided in at least in 3 languages: Hebrew, Arabic and English.
About the author:

My Name is Dalia Givony and I am a regulatory and clinical affairs consultant with 20 years of experience in global regulatory and clinical studies of medical devices, including 7 years at Medtronic specializing in executive RA & Clinical management. I offer my customers professional support for FDA submissions, CE marking, Global Registration (including registration in Israel), as well as design and management of clinical studies. With proven experience in direct communication and negotiation with the FDA, CE and other regulatory authorities, I aim to reduce the regulatory burden and V&V requirements and specialize in innovative, creative and effective regulatory strategies that take your other business aspects into consideration. Trust me to plan and manage cost-effective pre & post market clinical studies that will support your local regulatory requirements, as well as expansion into new markets. If your company needs regulatory and clinical support in Israel, you are welcome to contact me at [email protected] or visit www.daliag.com
Get the latest insights delivered to your inbox
Maximize Global Employee Engagement and Retention

Few companies expand their overseas workforce with a global HR strategy in place. Usually the process of going international is incremental, driven by overseas acquisitions, opportunities and new partnerships, rather than a strategic human resources plan. Whether or not you have a formal plan, clear communications, through localized onboarding, will help with your expanding roster of new employees must be a top priority.
In fact, distributing versions of localized onboarding materials and important internal communications is a legal requirement in many countries — and it is also an important indicator that you respect and value your entire international team. (It goes without saying that safety documents must be translated). This is where an effective HR team comes into the picture: by ensuring that your corporate communications and training programs are translated into the local language of all your employees, you can have a profound impact on employee satisfaction, engagement and loyalty. Here’s how you can get started:
Expansion & onboarding
When a company expands into new offices, factories, warehouses or distribution centers abroad, it needs to fill those new locations with qualified employees. HR is usually tasked with finding, signing and onboarding these new people — a task which inevitably involves a barrage of paperwork that must comply with local labor laws. For example, many countries require HR documents to be submitted in the relevant local language/s to government, labor, tax, social security and data protection authorities. In other countries, English-language HR documents are not necessarily prohibited, but they may not be legally enforceable. As such, you should always consider translating employee contracts, handbooks and training documents for non-native English speakers.
Training & eLearning tips
In a global business environment, HR teams are often responsible for creating localized onboarding programs for different roles and departments. One way to make training more effective is to produce online courses and videos that can be localized for employees who hold the same or similar positions in different countries (and therefore require the same training but in different languages). When you start working on a new training course, follow these three tips to make future translation attempts easier:
- Consider the reading level of your target audience: Are you training a financial analyst with an MBA, a factory manager with a high school diploma or a delivery and assembly team with little or no formal education? Be sure to create content that is at the appropriate comprehension level for each position you’re training.
- Write your course with clear and concise sentence structure. Try using lists to write step-by-step instructions that are easy to follow and digest — and never use the passive voice!
- Avoid culture-specific examples, comedy, slang, metaphors and abbreviations. This will make it much easier later on to convert the course into other languages.
Corporate communications
For your company to become a strong, well-integrated global organization, it’s important to communicate with your international employees in their own language. Your message and brand can easily get lost in translation if your internal corporate communications aren’t localized correctly. This can have a direct impact on your team’s creativity, effectiveness and engagement. And remember: In some countries, English-only communications can be a legal problem if national language laws or mandates exist. In France, for example, the national labor code punishes employers with heavy fines if they issue certain HR communications in languages other than French.
Providing accurate translations of HR communications is difficult enough without the additional burden of keeping track of the ever-changing global legal landscape. That’s where an experienced language solutions provider (LSP) can step in to help. An LSP can ensure that you are complying with worldwide language laws, while skillfully localizing global corporate communications to achieve a cohesive and successful international business network.
Platform localization
Your internal business software and operating platforms are only as effective as the people using them. If you want to expand your business into non-Anglophone countries, you need to consider who will be using your internal software and platforms there. If your international employees have difficulty understanding menus, buttons or dialog boxes, they are less likely to accurately use all the internal software features available to them. Localizing your business software for your international sites will result in higher comprehension of the tools available, which should ultimately lead to more efficient use of employee time.
Software localization includes the formatting of numbers & dates, the adjustment of sort orders, and the adaptation of fonts and images to local norms. It’s important to note that software localization can sometimes result in text expansion or contraction within the user interface (UI), in which case it’s important for your LSP to work with your design team to ensure that the final, localized UI is seamlessly presented to the end-user.
Embrace the opportunity
Localized onboarding offers a chance to strengthen employee engagement and company cohesion. Clear, consistent messaging that’s been optimized to resonate with employees around the globe can do wonders for your brand and your bottom line. Ultimately, the successful execution of a global HR localization strategy can position your company for game-changing growth. Consider the possibilities.
Get the latest insights delivered to your inbox
Why a Centralized Translation Source Matters for Biotech and Pharma IP

The biotech and pharmaceutical industries both work at the cutting edge of science to deliver medical innovations to patients around the world. While the intellectual property portfolios of each industry vary widely, the path to obtaining strong IP protection is similar. To recoup R&D investment, both industries need to file their parents broadly and on a global scale, and that path includes extensive patent translations.
What’s the difference?
Biotech & pharma are often used interchangeably but it’s important to understand the distinction. So before we cover the similarities between translation requirements for IP in the biotech and pharma industries, let’s take a brief step back to understand what types of products each industry covers:
- The pharma industry regularly seeks patents for new chemicals, pharmaceuticals, drugs, reformulations of existing medicines, as well as innovative manufacturing methods.
- The biotech industry is more wide-ranging with patents covering biological materials such as proteins, DNA, RNA, cell lines, hormones, engineered tissues, and artificial body parts. Biotech process patents can also include methods for working with DNA, proteins or cloning.
The question of patentability
It’s important to note that the nature of biotech innovations, especially those involving animals, plants and DNA, often makes them more challenging to protect because of the ongoing question of their patentability. In addition to the three global patent standards that an invention must be novel, non-obvious and useful, inventions must also cover a subject matter that is eligible for patent protection.
Many countries around the world are debating whether or not biotech innovations can be claimed by individual human beings or companies, which makes the international landscape for biotech patents extraordinarily complex as each nation works out its own set of rules and regulations.
Similarities in product development
Regardless of where you’re planning to file a pharma or biotech patent application, you will be required to provide extensive supporting documents about each phase of product development in that country’s local language. This continues after the patent has been issued and commercialization begins. A translation flaw anywhere along the way can slow down or even derail the entire process. For example:
- Preclinical studies — This phase often requires translations of internationally sourced case reports and data sheets before clinical trials can begin.
- International clinical trials — This phase requires expertly localized informed consent documents, personal information forms, case report forms, physician protocols and notes, and patient-reported outcome forms. Additionally, live interpretation services may be needed during this phase to help international teams communicate.
- Regulatory approval — Accurate and comprehensive translations must be submitted to national or regional patent offices, as well as other regulatory agencies, in regions where distribution of the product is anticipated. This includes dossiers, labeling, adverse event notifications, and other types of supporting documentation.
- Manufacturing — Documentation for Standard Operating Procedures (SOP), as well as health and safety materials, must be localized for each country or region in which the product will be manufactured.
- Marketing and sales — Customer-facing materials must be translated for each different market, expertly leveraging a deep knowledge of the local language and culture. This phase includes translating product websites, brochures, displays, advertisements, and other promotional materials.
Why a centralized translation source matters
It can take over a decade for a pharma or biotech innovation to go through all the phases of product development before it actually hits the shelves in your local pharmacy. Having a single language service provider (LSP) responsible for coordinating and delivering all of the various translations required throughout the product lifecycle guarantees a level of consistency and accuracy that simply can’t be replicated by the work of multiple independent agencies strung together along the way.
In addition to improving translation quality, using a singular LSP can save you time. Think about it this way: Instead of introducing and explaining yourself, your company and your invention to numerous agents repeatedly over what could be a 10+ year process, an LSP can set you up with a dedicated project manager who acts as your single point of communication for years to come. If you have questions, need clarifications, or require late-stage changes, the dedicated project manager is trained and positioned to shepherd your project to a successful conclusion.
Start early and start right
Now that it’s clear why an LSP is beneficial for biotech and pharma companies, let’s discuss how to choose one. First, the best time to start looking for an LSP is before entering the preclinical studies phase. This allows you and the LSP time to create and implement a clear project lifecycle calendar. Of course, an LSP can start working at any phase of product development, but it will be less stressful for you knowing it’s all taken care of from the start.
When selecting an LSP, you can check the credibility of an organization by asking about its ISO certifications. These are internationally recognized standards of excellence written by the independent, non-governmental International Organization for Standardization (ISO). There are literally thousands of ISO certifications to choose from, which (understandably) can seem a bit overwhelming. Here are three of the most useful ISO standards to look for in the language services industry:
- ISO 9001 — This certification means that a company meets international standards for maintaining a quality management system.
- ISO 13485 — This certification is based on ISO 9001 but adapted specifically for the medical device industry, with an emphasis on risk management and strict quality control.
- ISO 27001 — This certification means that a company meets international standards for information security management. Its primary goal is to help prevent data theft, loss and corruption.
Cost-saving tools
Once you’ve found an LSP you trust, be sure to notify every relevant department in your company as to your choice. This will ensure that you consistently benefit from the quality control and cost-saving tools available to you. For example, biotech and pharma documents contain a great deal of repetitive text. Certain professional translation tools exist to cut out redundant work and increase consistency across projects. These tools are especially handy for families of related products:
- Translation memory (TM) — This tool creates a database of previously translated text segments from which human translators can retrieve and re-use words, sentences, and paragraphs.
- Terminology management and glossaries — Every company has its own terminology, branding, and way of expressing itself. To ensure the consistent use of language across all projects and documents, an LSP can construct confidential, client-specific glossaries of your organization’s terminology which translators can utilize while working on your projects.
Additionally, check to see if the LSP offers an online client portal where you can manage all of your company’s projects, submit new ones and leverage historical data like glossaries, style guides and TMs.
Why make things harder?
The documents required for biotech and pharma patents, regulatory approvals, and marketing are complicated and must be impeccably executed. Quality is king in these industries and either one cannot abide costly, potentially even dangerous, errors in translations. Years of research, millions of dollars and lives are literally at stake – which is why the alternative to using a centralized translation service can quickly border on the nightmarish: identifying, hiring, managing and also verifying qualified service providers in each and every single region or country in which a product is to be sold or where its ownership must be protected. The redundancies are stupefying. Instead, with a centralized translation service, a single good hire of the service itself ensures consistency, quality and timely delivery for years to come.
Get the latest insights delivered to your inbox
Press Release: Morningside Acquires Life Science Language Services Leader Net-Translators

NEW YORK, NY – Morningside Translations, a Top 25 global provider of language services, recently acquired life sciences specialist Net-Translators. Headquartered near Tel Aviv, Israel, Net-Translators provides high-quality translation, localization and multilingual testing services to leading global medical device manufacturers, pharmaceutical companies, life science research organizations, and software developers.
The addition of Net-Translators dramatically expands Morningside’s global life sciences practice and significantly extends its life science reach across Europe, the Middle East, and Asia-Pacific. Morningside is already one of the largest global providers of IP services to the life sciences sector and a major partner to life sciences companies for their regulatory and clinical translation needs.
“We are thrilled to bring Net-Translators into the Morningside fold. This acquisition perfectly fits our DNA as a language service provider focused on regulated industries where quality is paramount,” said Morningside Translations’ co-CEO, Roland Lessard.
As the Tel Aviv branch of Morningside, the Net-Translators team will continue to provide the same exceptional quality and service to clients, while also leveraging Morningside’s broad international presence and top-tier network of linguists and subject matter experts.
The addition of Net-Translators also makes Morningside one of the largest language service employers in Israel, where it has had a robust presence since 2006.
“This is a natural extension for us across EMEA and Asia where our patent and legal work is flourishing,” said co-CEO Tom Klein. “Clients in the U.S. and overseas are always asking us where else we can help them across their broader business portfolios. We’re proud to now offer expanded, specialized capabilities.”
About Morningside Translations:
Get the latest insights delivered to your inbox
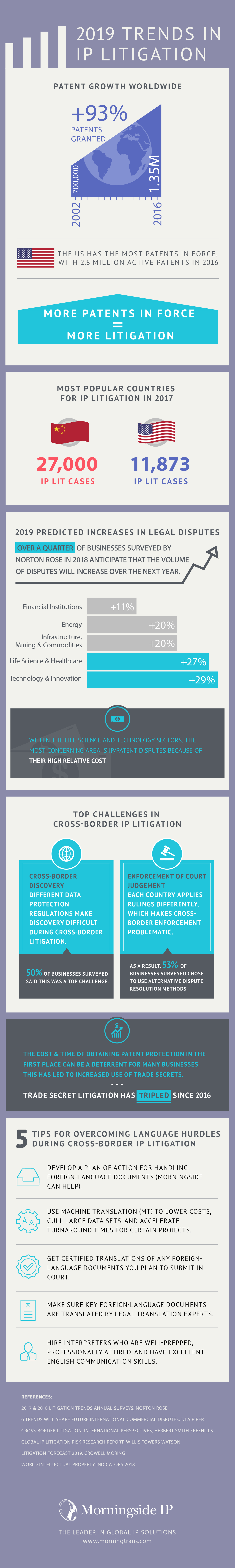
Infographic: 2019 Trends in IP Litigation

Innovation is on the rise, with a handful of industries — namely IT, telecom and life sciences — driving patent growth worldwide. According to WIPO, “Patents granted by the EPO grew by 40% in 2016 – the fastest growth since 1983.” In 2016, the Chinese patent grant rate increased by 13% and in Japan and Korea it rose by 7%. As the number of granted patents grows around the world, the number of IP litigation cases has also grown – which is a serious concern for businesses and patent owners, given their high costs. Check out our infographic below to learn more about the latest trends in IP litigation and to get tips for overcoming IP litigation challenges.