In the medical device industry, product and industry-specific terminology are woven into customer-facing materials, from software, technical documentation, and marketing materials to websites. It’s also riddled with internal documents like training materials and MSDS. So, in order to ensure the highest quality medical translation of these materials, terminology management is a must.
Terminology management starts with authoring, meaning that it needs to be consistent in the source content to provide even the most qualified medical translation partners with the best possible start to a project. The terminology needs to be cataloged with term bases and glossaries and communicated to translators so words and phrases are used consistently. Failure to do so can easily lead to confusion on the part of the translator and, ultimately, the end-user.
Here’s an example of a simple term that illustrates how complex it can be. Here are four definitions of the word “strike” with different meanings, along with the translation into Japanese. Notice that the parts of the speech vary, as does the context around the definition of the word.
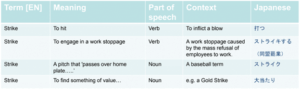
Now, it may not be too hard to determine which definition of the word is being used in English. However, there’s no doubt that it will be harder to determine in other languages, especially if there’s no context. Context is critical in understanding what a word means, and context must be included in a terminology management system. If you look at the Japanese translations, you’ll get a completely different understanding of the complexity involved. Each word is obviously other and therefore needs to be clearly defined.
The example of word “strike” is just a simple example. Many industries, such as the medical, legal, and manufacturing industries, have much more complicated terminology. If the terminology isn’t correct in the source language, let alone in the translated versions, it can have dire consequences. And in the case of medical translation, it could even potentially cause death. It’s critical to manage terminology, and there can never be too much planning. In this article, we’ll cover some best practices for managing terminology.
Define Terminology in the Concept Stage
Ideally, you should think about terminology when you’re in the concept stage of a project so that everyone involved in the project can be consistent when developing the product and associated materials. A group of people, including technical writers, developers, training staff, QA testers, marketers, linguists, etc., should be involved in making terminology decisions. Everyone will have a different viewpoint, and through discussions, you can decide on what makes the most sense for the audience and industry.
How to Get Started
You can use a variety of tools to track your set of terms. Many companies use a simple Excel spreadsheet; you don’t need to use a fancy tool. One thing to consider is what kind of metadata to include to manage terminology internally in the best way possible and to help the linguists. ISO 1087-1:2000 provides guidance on terminology management. Per this standard, the minimum terminology requirements to include are:
- Entry number
- Preferred term(s)
- Admitted term(s)
- Deprecated term(s)
- Definition
- Example(s)
- Note(s)
Again, context is critical for linguists to understand a term and then translate it appropriately. There are many other types of metadata that you can consider depending on the source material, your industry and your business. Some additional things to consider include:
- Part of speech
- Status
- Comment(s)
- Audience
- Protocol
- Application
- Component
- Location in product/document
- Picture/graphic
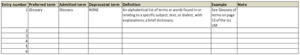
Here’s an example of a simple starter glossary that uses the following metadata: entry number, term, admitted term, deprecated term, definition, example and note.
Engage Your Medical Translation Provider
You know your products the best, so extracting your terms is best. However, another option is to have your medical translation provider develop a glossary for you. This option still involves collaboration and input from your internal teams.
Here’s a brief sample process workflow:
- Your medical translation provider will create a glossary based on extracting term candidates and add metadata.
- They’ll send it to you to approve and get feedback.
- The translators will translate the terms.
- Your medical translation provider will send the translated terms to your in-country reviewers (ICRs) to get feedback and approval.
- They’ll convert the terms into a term-base and distribute it to the translation team to use.
Starting in the Middle: What to Do?
So, what happens if you’ve already translated some content but you haven’t done anything with terminology yet? It’s not too late to start, although it will require some work. You or your medical translation services provider will need to create the glossary of terms. Then the translation company will have to do a consistency check on the translation memory (TM), which is where the translated content resides, to see if those terms have already been translated. If so, the linguist will validate the translations and get approval from the ICRs. If not, the translators will translate the terms and then get approval from the ICRs.
Getting Started the Right Way
There’s no such thing as too much upfront planning in the world of medical translations since mistranslations can have drastic consequences. So start with a self-assessment. Is there any terminology in place? How is it being managed? What tools are being used? And, how is it being communicated to internal users and translators, and also external translation providers? Ask the questions and make sure they all of the key stakeholders are on the same page before any new content is authored. Quality translations start at the source, so take the time to ensure there’s a solid foundation in place for consistent, repeatable and accurate content.
Need help getting started? Morningside has been providing language asset management services to medical device companies for over 20 years. Contact us if you have any questions or want to partner with an expert.