Year: 2020
How Technical Translation Services Help Move Manufacturing Products

In today’s global economy, manufacturers often need to prepare technical translations of product instructions (i.e. MSDS’s, technical manuals, user guides) in multiple languages for markets around the globe. This is necessary when a single country has more than one national or commonly spoken language, such as English and Spanish in the United States, or when a company is trying to break into a new foreign market. When translating technical documents, the core of the task is to faithfully convert the words from one language into another so that consumers and local vendors clearly understand how a product works. To guarantee that translations successfully convey the intended meaning, best practice requires the use of professional technical translation services with technical reviewers who are also subject matter experts (SME). An SME helps ensure the accuracy of the final technical translation (e.g. user manual or operator’s guide), and can also help identify issues that may exist with the original documentation.
Engineer-written documentation
Manufacturers are increasingly asking engineers to write user instructions for the products they’ve developed. On one hand, this makes perfect sense. The engineers know the products inside-out, so they can quickly put the instructions together, and there’s no need to spend additional money outsourcing the project to technical writers. On the other hand, a technical document produced by engineers can prove more difficult for the end user to understand and follow. Here’s how:
- Accuracy – The engineer’s area of expertise is engineering – not writing. Engineer-written text may contain grammatical errors or irregularities that even the most determined consumer will struggle to understand.
- Exclusion – It’s not uncommon for an engineer to consider a product’s features and uses to be self-evident, because, to an engineer, they are. It can be difficult for an engineer to accurately identify all the features and concepts that require explanation for an end user.
- Jargon – Engineers may use terminology that a user will not easily recognize.
Improving user manuals with SME’s
Technical translation services can connect you with an SME who is capable of extracting the intended meaning from the original document and deliver it with enhanced clarity in the target language. He or she will understand the subject well enough to recognize when an explanation is insufficient or irrelevant for end users, and can make any necessary refinements. Essentially, an SME translator may need to perform two technical translations:
- one from engineer-speak to user-speak; and
- one from the original language to the target language.
A strong relationship that allows two-way communication and collaboration between the manufacturer and the technical translation service can significantly streamline the document translation process. Technical translation services can help by:
- Providing instruction for engineers who are writing user manuals, or assisting them in the use of Simplified Standard English (STE), a form of writing designed to produce translation-ready text.
- Making sure the engineers have the tools they need to produce a consistently formatted original that follows the manufacturer’s style guide.
- Cleaning up original text to resolve any potential problems prior to technical translation.
Translating text within technical art
An especially difficult issue when translating user documentation is translating the text within technical art, such as figures, diagrams, illustrations, or any other type of embedded graphics. These may have been authored in specialized formats produced by computer-aided design (CAD) software, vector illustration, spreadsheets, or photography applications. A technical translation agency can help devise strategies for translating such text and meticulously preserving its meaning in the target language.
Good technical documentation results in a better user experience
Ease-of-use of a manufacturer’s product is one of the critical ingredients in a positive customer relationship. This leads to repeat business and recommendations that help the manufacturer maintain a reputation for excellence. Putting effort into high quality technical translations that help ensure a rewarding user experience is more than just something a manufacturer is expected to do — it’s a shrewd long-term strategy for success in international markets.
Get the latest insights delivered to your inbox
Corporations: Why You Need To Consolidate Your Third-Party Translation Costs

It isn’t uncommon for international corporations to employ an assortment of law firms or outside counsel to meet different local and regional legal needs. In doing so, a significant amount of money will inevitably be spent on legal translation strategy and services for cross-border or multi-country dealings.
Often, corporations leave these legal translations to their outside counsel to handle. While this may seem the simplest solution, it forfeits an opportunity to simultaneously keep costs under control, improve efficiency and data security, and ensure absolute quality across all legal translations. This is where a centralized language service provider (LSP) can make all the difference.
Three downsides to uncoordinated legal translations
A corporation that allows outside counsel to handle its legal translation work faces three distinct challenges:
- Cost — We know of corporations whose network of third-party attorneys contracted up to 140 different translation services in 2021 alone. That’s a significant annual spend! Unfortunately, with translations spread out this way, a corporation has no visibility into cumulative expenditures – let alone an opportunity to benefit from the discounts that such a hefty billing should merit.
- Quality — With each law firm (presumably) vetting and hiring its translators, the corporation cannot guarantee consistent terminology or accuracy in the translations upon which its business activities heavily rely. In addition to bearing the potential for undermining the success of current activities, inaccurate translations in legal documents can lead to significant problems in the future and potentially hurt your corporation’s reputation. As it has been proven time and time again, word choice can mean everything in litigation, and with each local law firm hiring a different translator, the corporation lacks the means to mitigate such risks comprehensively.
- Vetting — Lastly, there is no way to be confident that third-party agents are selecting translators strictly based on quality and pricing and not due to some unstated relationship that has nothing to do with the corporation’s interests.
Five benefits of a consolidated legal translation strategy
Corporations that choose to consolidate their legal translation services under a single LSP benefit in the following five ways:
- Translation projects gain enhanced transparency — On a micro-level, the corporation gains the ability to track the progress of translations during production and to make last-minute changes should a legal strategy shift occur. On a macro level, the large amount of work being tracked across the organization, when combined, can qualify for significant cost savings.
- Accountability becomes straightforward — By selecting a certified translation service with rigorous internal quality control processes, the corporation recovers control of translation quality, thus ensuring that all legal translations are produced at the highest level and with the maximum protection as they guard against future issues.
- The corporation gains control of consistency — Corporations can work with LSPs to create customized glossaries that will ensure the constant and correct use of preferred terminology for products and other standard references in every relevant language.
- Translation memory discounts — Over time, an LSP will be able to incorporate dedicated translation memory tools for further cost reduction through efficiently re-using previously translated material. The longer the relationship between the corporation and the LSP, the larger the company’s translation memory grows and the greater the savings that can be achieved.
- Centralized & easily repeatable processes — An LSP’s account services may be able to lead the corporation through each project, consulting on best practices and providing advice that helps ensure friction-free, strong, and successful legal translations.
Taking control of translations
International corporations who use outside counsel can make use of these benefits without causing any complex internal changes or creating any additional work for counsel. Choose a reliable and experienced LSP to handle organization-wide translations and then tell each law firm under contract to obtain all future legal translation services from that LSP. Outside counsel can instruct the LSP on your behalf, which will immediately achieve greater efficiency, consistent quality, and cost savings for your corporation.
About Morningside
Morningside, a Questel Company is a recognized leader in comprehensive legal language solutions and eDiscovery services for the legal industry. We provide the world’s largest law firms and corporate legal departments with a full suite of services, ranging from legal document translation, contracts and compliance documentation to full-scale multilingual litigation requiring certified translation and foreign language document review to supporting complex eDiscovery projects. We deliver customized legal document translation solutions based on your case’s size and budget requirements, utilizing industry-leading technology to ensure accuracy, lower costs and faster turnaround times. Find out why 97% of the Am Law 200 and 90+ leading global brands rely on Morningside.
Get the latest insights delivered to your inbox
Applying for a Patent in South Korea

As one of the world’s most vibrant markets, the number of patents granted in South Korea by the Korean Industrial Property Office (KIPO) has been steadily rising. In just a single year, 2018, patent filings increased by nearly 5% over the previous year – and 3.6% of these (47,410 applications) were filed by international claimants.
If you wish to join the trend and patent your innovation in South Korea, it’s critical to understand how the South Korean patent application process works. For example, did you know that you must submit a Korean translation within 31 months from the earliest priority date if you’re filing a PCT application, but you only have 14 months if you’re using the Paris Convention route?
Learn more about the filing and examination process in South Korea by viewing our most recent article on IPWatchdog. There, we cover:
- Patentability in South Korea
- Available filing methods
- Korean language requirements
- Time and cost involved
About the authors:
This article was co-written by John Harris at Morningside and Jongseop Yun at AIP Patent & Law Firm.
Get the latest insights delivered to your inbox
Applying for a Patent in Germany

Did you know that Germany is now ranked as the most innovative nation worldwide? Bloomberg reports it’s a thriving European center for innovation where patent activity, high-tech density, and value-added manufacturing are on the rise.
If you wish to join the trend and patent your innovation in Germany, it’s critical that you understand how the the German patent application process works. For example, did you know that filing your patent application with the German Patent and Trademark Office secures your priority date – but it does not begin the official examination required to grant the patent?
Learn more about the filing and examination process in Germany by viewing our most recent article on IPWatchdog. There, we cover:
- German patent filing methods
- How to begin examination in Germany
- Germany’s language requirement
- The difference between German patents and utility models
About the authors:
This article was co-written by Morningside and Stolmár & Partner IP.
Get the latest insights delivered to your inbox
5 Tips on Translating Medical Device Labeling

Regulatory authorities around the globe view medical device labeling as an integral part of the medical product itself. These documents provide critical risk/benefit information as well as clear instructions for safe use. They come in a wide range of formats, including brochures, leaflets, user manuals and videos – basically, any document containing explanatory information geared toward the patient.
When a medical device is introduced to a new market, all labeling content is carefully reviewed for compliance with local rules and regulations. Any problems with the way the labeling is worded could result in a delay in distribution, a product recall or adverse events with patients. That’s why it’s critical that these documents – and all medical translations of them – are as accurate as possible from the beginning. So, check out the following five tips to learn more about overcoming multilingual issues in medical device labeling.
Tip #1: Use accessible language
The efficacy and safety of a medical device is dependent on proper usage. Be sure to avoid jargon and keep instructions simple. Not only will this kind of language make it easier for English-speakers to understand, it will also make it easier to translate medical labels into the other languages required.
Tip #2: Provide documentation electronically
Some regulatory authorities require medical device labeling to be provided online, and it should be clear to the end-user where that information can be accessed. Digital deployment makes it easier to provide labeling in multiple languages without adding bulky documentation to the device packaging. It also makes it easier to keep information up-to-date.
Tip #3: Use graphics liberally
Medical device labeling is a classic case where a picture is worth a thousand words when incorporated effectively. Try making graphics large enough to convey the focal point. Use dark, sharp lines for good contrast, and add cues such as circles or arrows to highlight key information.
Tip #4: Use internationally recognized symbols and icons
Many symbols and icons have become standardized and should be used wherever possible in medical device labeling to overcome language barriers. However, it is incumbent on the manufacturer to ensure that the symbols and icons will be properly understood by any given target audience – which is why pretesting and localizing of medical labeling content is critical.
Tip #5: Use videos or animations
Supplement classic instruction manuals with engaging videos or animations that contain minimal text and maximum imagery.
Start early, finish ahead
The complicated process of medical device labeling is easier to manage if its multilingual components are addressed strategically from the beginning. Clearly translated medical content and a rich array of graphical elements will ensure that the labeling makes sense across a wide range of target languages. If you need help translating your medical device labeling, reach out to Morningside below. Our team has in-depth industry knowledge of country-specific regulations, global markets, and the entire life sciences commercial supply chain.
Get the latest insights delivered to your inbox
All You Need to Know About Text Expansion and Contraction

You may have noticed that it can take more words to say something in one language than in another. For example, it takes three words in French (s’il vous plaît) to accomplish what you can with one word of English (please). Text expansion and contraction has a direct impact on the cost of translation, and it plays an extremely important role when considering design for multilingual websites, marketing campaigns, presentations – and pretty much any project that needs to be available in multiple languages.
What is text expansion and contraction?
This is a term used in the translation industry to account for the increase or decrease in a document’s final word count when it is translated. For example, French and other romance languages are known to be wordier than English. A document with 1,000 English words translated into French will convert into approximately 1,150 target words — a 15% increase in the document’s word count. That is text expansion. Asian languages like Chinese, Korean and Japanese will usually convert into fewer total words when translated into English. That is text contraction.
How does text expansion and contraction affect pricing?
Calculating expansion or contraction during translation is not an exact science. Most language service providers use ratios based on typical expansion/contraction rates for different language pairs. For example, in English to German translation, the word count usually contracts by 20%, and therefore this is the ratio that most translation companies use. (If you have ever seen a German compound word like Donaudampfschifffahrtsgesellschaftskapitänsmütze – which translates to “Danube steamboat shipping company Captain’s hat” – you’ll understand why.) Pricing can also be affected by a document’s subject matter, terminology, and the quality of the original writing — all of which can cause the text to expand more or less than the ratios predict.
How does text expansion and contraction affect design?
Let’s say you have a short product description in English (100 words) that needs to be translated into French for a PDF brochure, a video advertisement, and a software app. Here’s what could happen: A one-line English headline in a cleanly laid out PDF brochure can turn into a two-line French headline that bumps the rest of the copy down or even off the page. A video with music and graphics synced to English text can become unsynced when the text is translated to French. The menu buttons on a software app can end up expanding awkwardly or displaying incomplete words, causing a major UI problem. So, as you can see, translations that result in text expansion or contraction can have a very real impact on design quality. That’s why it’s important to work with a localization expert to ensure all of your materials are not only translated accurately, but visually adapted as well.
Trust a localization expert
Morningside has 20 years of experience working with businesses to linguistically adapt products, documents, websites and software applications for target markets around the world. It’s a process we call localization, where translation is just the first step. We then work to adapt layout, design and graphical elements to properly fit the translated text. Our team includes expert linguists, DTP specialists, voiceover talent, and dedicated project managers experienced in providing multilingual localization services in 200+ languages. If you have any more questions about text expansion and contraction, or localization in general, contact us below.
Get the latest insights delivered to your inbox
3 Simple Interpretation Solutions for Social Distancing

Over the course of months of worldwide social distancing efforts, inquiries regarding Morningside’s interpretation capabilities have increase dramatically and we’ve seen a shift in the way that clients are utilizing our services. We understand that our clients need greater flexibility during this time, and want to confirm that Morningside’s interpretation capabilities are readily available to you for depositions and court proceedings, as well as mission critical business functions. You can read more about them below.
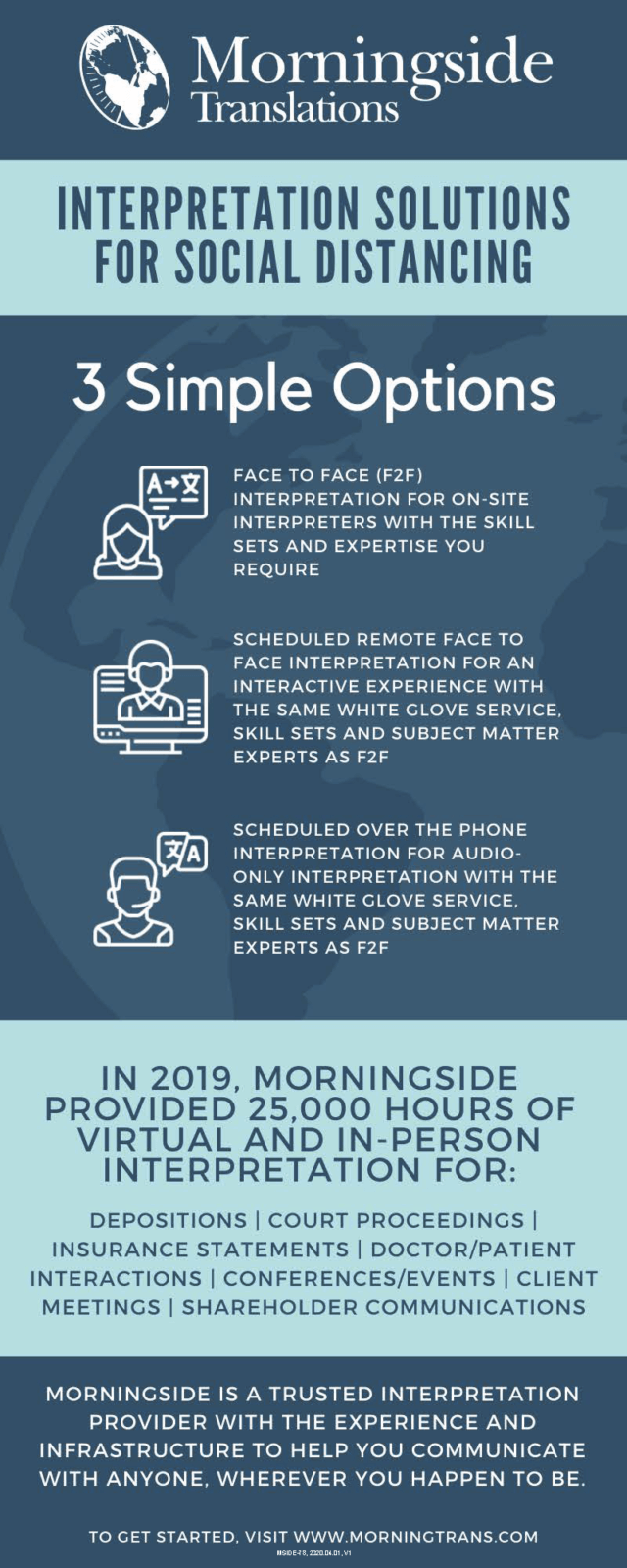
Get the latest insights delivered to your inbox
Conducting International Clinical Trials During COVID-19

With people everywhere anxiously awaiting word of an effective treatment or vaccine for COVID-19, clinical trials have never before been so closely watched by so many. There are, according to one count, over 1700 novel coronavirus-related clinical trials underway around the world as of this writing.
Fast track approval
When a drug is urgently needed for a serious, currently untreatable disease, such as COVID-19, it may be possible to gain “Fast Track” approval, an emergency-case designation awarded as necessary by the FDA in the U.S and by other nations such as China and the U.K.
This recently occurred with Gilead’s drug remdesivir, which had already gone through clinical trials for treating Ebola. The Ebola trials ultimately proved disappointing for remdesivir’s efficacy in fighting that disease, though there were no questions regarding its safety. When COVID-19 arrived, Gilead revived remdesivir as a drug candidate because it did seem able to inhibit SARS-CoV-2 in vitro.
Granting fast-track approval for a COVID-19 drug that was known to be safe was an easy choice for the FDA, and remdesivir was made available for use by emergency physicians treating COVID-19 patients. The results, however, are mixed: While some limited data suggests remdesivir may shorten the duration of the illness, a large Chinese study of patients with more severe cases of COVID-19 found no statistical benefit from the drug.
Clinical trial documents
Pharmaceuticals are, of course, a field where the smallest details matter, and nuance can be everything. One of the trickiest, most critical, parts of international clinical trials is making sure that everyone involved shares a common understanding of the clinical trial process and its findings. That is why clinical trial document translation is required by law across the globe.
Every trial participant must be able to access all materials in their native language in order to avoid errors and invalid results. It’s also important to translate all documentation for those who will be planning, administering, and conducting the trials. This is the best way to ensure that no misunderstandings occur between parties operating in different regions, and that important implications and subtleties in one language are faithfully communicated to everyone involved.
How an LSP can help
When clinical trials are conducted internationally, clinicians, patients, sponsors and regulatory bodies must be able to communicate easily and with a high degree of accuracy. Engaging a qualified language service provider (LSP) such as Morningside increases the likelihood of better outcomes, ensuring that multilingual communication never stands in the way of a trial’s productive completion.
A good LSP can also help to quickly move a study forward by providing rapid turnaround of the necessary translated documentation, allowing medical research teams to meet critical regulatory deadlines. With the added pressure of finding a vaccine for COVID-19 as quickly as possible, quick turnaround is more important now than ever before.
If you are involved in COVID-19 clinical trials, feel free to contact us about how to achieve the best translation results. We are experts at translating and validating a wide range of clinical trial documents — from informed consent forms (ICFs) to questionnaires, COAs of all types and case report forms (CRFs). Our team of linguists consistently deliver accurate, culturally adapted translations for clinicians, review boards, and regulatory bodies.
Get the latest insights delivered to your inbox
The Challenge of Multilingual Documents In E-Discovery

The rapid increase in globalization and international commerce means that more and more disputes are crossing borders and jurisdictions. In a recent survey of multinational corporations, almost 50% experienced an increase in cross-border litigation during the past 2 years. The volume of electronic information has also grown exponentially with more people producing more data – on their computers, the cloud and with mobile devices. Technologies for creating and storing electronic data are evolving quickly, adding to the challenges of e-discovery.
On par with changing technologies, the legal industry and discovery, specifically, have also shifted. Computerized information has created mountains of data for legal teams to sift through during e-discovery. Complicating matters even further, cross-border disputes often involve documents in multiple languages. The need to review, organize and translate these foreign language documents can add significantly to the cost, complexity and lead time for e-discovery projects. English-speaking lawyers can’t even begin to review foreign language discovery documents until they are translated to English – potentially delaying the identification of key trial information. And strict budgets and tight deadlines place additional stress on legal teams already bogged down in the e-document mire. Sound familiar?
If you work at a law firm or as part of a corporate legal team, multilingual documents in e-discovery present unique challenges. The pure volume of documents alone can be overwhelming. This is where a language service provider (LSP) can offer some much-needed relief.
LSPs use specialized tools to cull large data sets into more manageable chunks of case-relevant information. The application of filtering techniques like foreign language keyword searches, for example, can identify and remove irrelevant documents from the data set. Then machine translation (MT) can be used to determine the “gist” of what each document contains, helping determine which ones require more accurate human translation.
Lawyers and firms who use kCura’s Relativity platform to manage their document review process can help streamline the translation of foreign language documents during their case. Language Connect, Morningside’s free Relativity plug-in, expedites the translation of foreign language documents by allowing users to instantly send multilingual files for quotes and language identification directly from their own Relativity environment. After choosing between human, machine or hybrid translation solutions in more than 200 languages, all completed translations are automatically delivered back into the Relativity environment – ensuring seamless delivery.
While the review phase of multilingual e-discovery poses unique challenges, legal teams that rely on Relativity can simplify the process by obtaining translations via the plug-in. It’s a process that not only saves time and money, but also grants peace of mind in a world of ever-increasing e-data.
Get the latest insights delivered to your inbox
EUMDR Update: Prioritizing the Fight Against COVID-19

On April 3rd, the European Commission adopted a proposal to delay the application of the EU MDR by one year, to May 26, 2021 in order to allow “Member States, health institutions and economic operators to prioritize the fight against the coronavirus pandemic.”
What does this mean for global healthcare?
This is a positive outcome for healthcare systems across the EU, as it provides for uninterrupted access to vital life-saving devices.
Vice-President for Promoting our European Way of Life, Margaritis Schinas, said: “Shortages or delays in getting key medical devices certified and on the market are not an option right now. The Commission is therefore taking a pragmatic approach and delaying the entry into application of new EU rules on medical devices, so we can have our medical industries pouring all their energy into what we need them to be doing: helping fight the pandemic.”
Stella Kyriakides, Commissioner for Health and Food Safety, added: “Any potential market disruptions regarding the availability of safe and essential medical devices must and will be avoided.”
What does this mean for manufacturers?
The delay is also a positive outcome for device manufacturers. First, it obviously means more time to become compliant. According to industry polls, around 40% of manufacturers weren’t going to be fully compliant by the May 26 deadline. Now there’s enough time to get processes and documentation in shape, including preparing all required translations.
Second, it means more access to notified bodies. There are only 11 MDR-designated notified bodies today. The extension should allow for more notified bodies and increased overall capacity.
Third, it means that manufacturers are able to prepare multilingual documentation for all of their target markets in the EU. There’s an enormous volume of documentation under the MDR that must be translated into the official language(s) of each country. Many companies were prioritizing languages in order to meet the deadline, but now there’s sufficient time to prepare documentation for all intended EU markets.
Stay current & keep in touch
For a refresher on the complex EU MDR language requirements, take a look at our recent webinar that we co-produced with RAPS.
If you have any questions about translating your documentation or MDR compliance in general, please contact us directly.


